While surveying across the country, we frequently see the same issues over and over again. Despite educating the Operating Room staff, specifically anesthesiologists and CRNAs, we continue to see the same deficiencies. This one is my personal favorite. 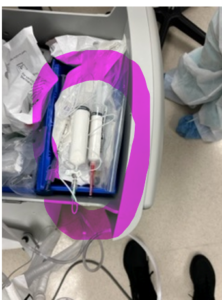
When opening up the anesthesia cart, low and behold, I find unlabeled, unsecured syringes with a white solution in them. When questioning the anesthesiologist/CRNA about what the solution is in the syringe I am told that it is Propofol. At that point, I make an attempt to educate the anesthesiologist/CRNA regarding the labeling of medications that are not being immediately administered after being drawn up and that the medication needs to be secured. Despite my best efforts, the response is, “Propofol is the only medication that looks like milk, I know what it is.”
Yes, we all know that Propofol looks like milk, however, the Joint Commission standard is very clear. MM.05.01.09 EP 1 - reads, “medication containers are labeled whenever medications are prepared but not immediately administered.” That being said, the next time you or your staff are doing tracers in preparation for your next survey, please check the anesthesia cart and the anesthesia medication cart. Continue to educate your staff related to safe medication management in the Operating/Procedure area environments, and the importance of securing them. Remind them that all medications that are not immediately administered need to be labeled per the standard. (MM.05.01.09 EP 3 - All medications prepared in the hospital are correctly labeled with the following:
- Medication name, strength, and amount (if not apparent from the container)
- Expiration date when not used within 24 hours.
- Expiration date and time when expiration occurs in less than 24 hours.
- The date prepared and the diluent for all compounded intravenous admixtures and parenteral nutrition formulas.)
It is important to note that Propofol is only good for 6 hours once transferred to a different container (ie: syringe) and only good for 12 hours once the original container is opened. Beyond that time, the risk of contamination, since it is egg-based, rises dramatically creating a patient safety issue. Always include the expiration date/time on the label to prevent this potential safety issue. Please refer to the package insert for stability information.
Please contact us for questions or more information at 704-573-4535 or info@courtemanche-assocs.com.
Courtemanche & Associates specializes in Healthcare Accreditation and Regulatory Compliance Consulting Services. With over 29 years of being in business and 100+ years of healthcare experience amongst our consulting team, we are ready to assist with your accreditation and regulatory compliance needs.

