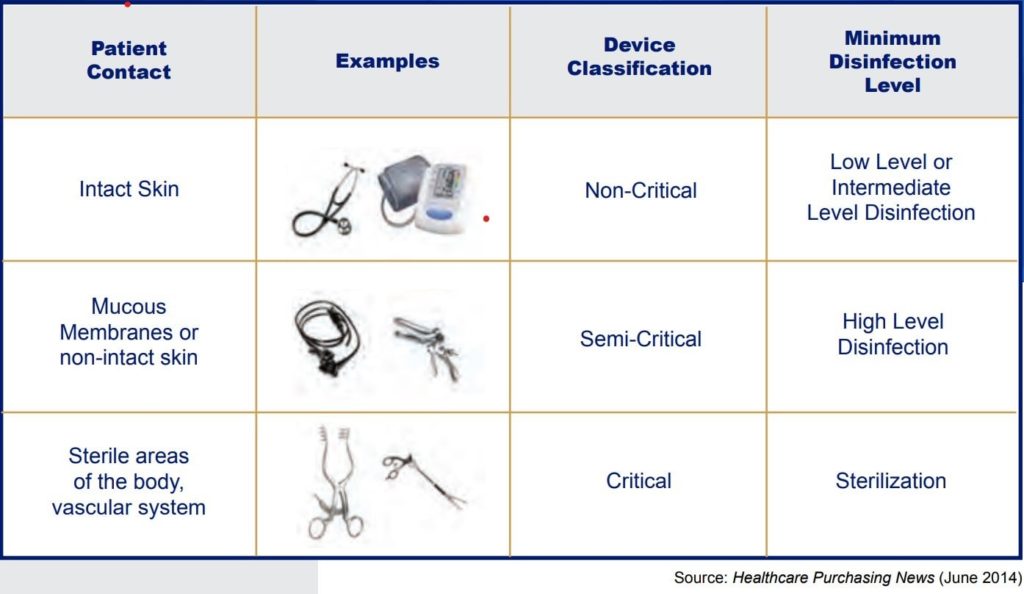
High-Level Disinfection is one of the top 10 TJC findings for 2021. More than 45 years ago, Earle H. Spaulding developed an approach to disinfecting and sterilizing patient-care items and equipment. His categorization has been successfully used by infection control professionals and others when planning methods for disinfection or sterilization. Spaulding believed that if instruments and devices for patient care were placed into different classifications according to the degree of risk for infection involved in using the items, this would guide how to disinfect and sterilize them. Below is the classification system he devised, known as the “Spaulding Classification.”
Even though this classification system has been in effect for more than 45 years, we still see breaches in the high-level disinfection process. The consultants at Courtemanche and Associates would like to take the opportunity to share with you the findings that we see from the field.
-
Failure to spray instruments at the point of use (POU) prior to sending them to be reprocessed.
- Before sending instruments for reprocessing, it is essential to remove visible blood and bioburden from the instruments and keep them moist until they are reprocessed to prevent any remaining bioburden from drying onto the instrument. While wearing proper PPE, first, rinse or wipe the instruments. This is usually performed with water but double-check the manufacturer’s instructions for use. Only use a sink that has been approved as a soiled sink for rinsing contaminated instruments. Never use saline, unless recommended by the manufacturer, as it will damage your instruments. With the instruments in their open position, spray them with an enzymatic spray or foam, ensuring that the instruments are completely covered with the spray or foam. The label on each enzymatic spray or foam product will indicate how long the product will remain moist. This is particularly important if instruments will be left unprocessed for several hours or overnight.
- Instruments are to be sprayed at their point of use and must be transported in a leak-proof, puncture-proof container with a lid and labeled with a biohazard sticker. Dirty instruments awaiting pick up or delivery to Sterile Processing must be stored within the dirty utility room.
- It is important to note that dirty, contaminated instrumentation cannot be carried in hand with a towel, glove, or biohazard bag to the Dirty Utility Room. This would be an infection control issue and a puncture risk.
- Do not forget to check the expiration dates on the enzymatic too. With the increased use of single use instruments, organizations are not using as much enzymatic spray which results in spray being found expired on the shelves.
-
Failure to properly transport instrumentation for reprocessing.
-
- For the Surgical Suite, once the instruments are sprayed with the enzymatic at the point of use, lay them in a leak-proof, puncture-proof container with a lid and a biohazard sticker. Hinged instruments should be in the open position. (String the instruments in the open position and spread them out to ensure complete coverage with the enzymatic). If a stringer is not available, the instruments must be layered in the container, ensuring that each layer is completely sprayed to avoid bioburden from sticking to the instruments. Make sure to minimize the splashing of liquid on transport.
- For the nursing units, transport them to the Dirty Utility Room in a leak-proof, puncture-proof container with a biohazard sticker and a lid to the container. Instruments should be in the open position.
- NOTE: While it is preferred that the instruments be in their open position, the instruments should never be touched to reposition them.
-
Reuse of single-use instruments.
- Single-use instrument means just that. Once you have completed the procedure, you are to dispose of that single-use instrument per your organization’s policy and procedure. Typically, single-use instruments are disposed of in sharps containers. As a side note, many single-use instruments are stamped “Pakistan,” these instruments are made from low-quality metal, and some processes like passivation (a widely used metal finishing process to prevent corrosion) are not done on these instruments. For single use, these instruments are acceptable as they are not reused and are inexpensive to buy; however, they serve the purpose at the time. These instruments should NEVER be reprocessed.
- Single-use instrument means just that. Once you have completed the procedure, you are to dispose of that single-use instrument per your organization’s policy and procedure. Typically, single-use instruments are disposed of in sharps containers. As a side note, many single-use instruments are stamped “Pakistan,” these instruments are made from low-quality metal, and some processes like passivation (a widely used metal finishing process to prevent corrosion) are not done on these instruments. For single use, these instruments are acceptable as they are not reused and are inexpensive to buy; however, they serve the purpose at the time. These instruments should NEVER be reprocessed.
-
Failure to inspect instruments.
- Part of the high-level disinfection process is the instrumentation inspection before high-level disinfection. It is essential to check the gears, hinges, and screws. If instrumentation is not working correctly or is loose, this can pose a safety risk to the reprocessing staff, physician/surgeon, and patient. Damaged or improperly functioning items need to be reported to the reprocessing department to be sent out for repair or replacement. It is expected lighted magnification is consistently used to ensure thorough inspection of instruments. Bioburden, damage, and other abnormalities can best be observed through lighted magnification where they may be missed through normal visual inspection. This inspection occurs within the Sterile Processing Department.
-
Failure to follow the Information for Use (IFU).
-
- There is no underestimating the importance of Instructions for Use (IFU) documents. The most current instructions are critical for patient safety in a life-or-death situation. The IFU provides detailed, action-oriented, step-by-step written, and sometimes visual instructions for the staff on using the equipment, including cleaning, handling, storage, and disposal. In accordance with the Medical Device Regulation, the term "instructions for use" refers to the information provided by the manufacturer to inform the user of a device's intended purpose, proper use, and any precautions to be taken. Every medical device has its own IFU. Even before a medical device can be sold, the Food and Drug Administration (FDA) requires validation testing to demonstrate that the device can be effectively cleaned, disinfected, or sterilized in healthcare facilities by following the IFUs.
- Enzymatic sprays and cleansers used during the point of use cleaning have IFUs as well. Remember to check those IFUs for proper use, mixing instructions, interactions with other solutions, and disposal.
- Checking your IFUs will give your instrument/devices life and save your organization money.
- IFU’s also need to be readily available to staff using the device or product. Many organizations will print them and place them in binders for staff to reference. Others have made laminated wall posters for staff reference. Computer access can be acceptable provided the staff are able to independently access them.
-
Failure to use the proper personal protective equipment when using enzymatic sprays and cleaning instrumentation and equipment.
-
- Each product Safety Data Sheet contains recommendations as to which Personal Protective Equipment should be worn when handling or using the product. Most enzymatic sprays recommend the use of eye covering and gloves.
- Gloves should be worn when performing point-of-use cleaning (wiping down and spraying with enzymatic product).
- The appropriate personal protective equipment (PPE) is required when decontaminating instruments in the reprocessing area and includes:
- Hair covering or cap
- Face mask/shield
- Fluid resistant gown
- Durable gloves that do not tear or leak when hands are immersed in water
In summary, high-level disinfection ensures the safe use of devices, classified as semi-critical according to the Spaulding Classification grid. However, it is essential to remember that before cleaning and disinfecting any instrument, it is necessary to refer to the manufacturer’s information to ensure effective and adequate disinfection and avoid damage to those devices. Do not forget to wear the proper PPE!
Patient safety is our top priority! By caring for the instrumentation from the point of use, our patients will stay safe, and your physicians will be happy.
For any questions regarding high-level disinfection, contact us a Courtemanche and Associates.
For more information on High-level disinfection check out the Quality Academy.