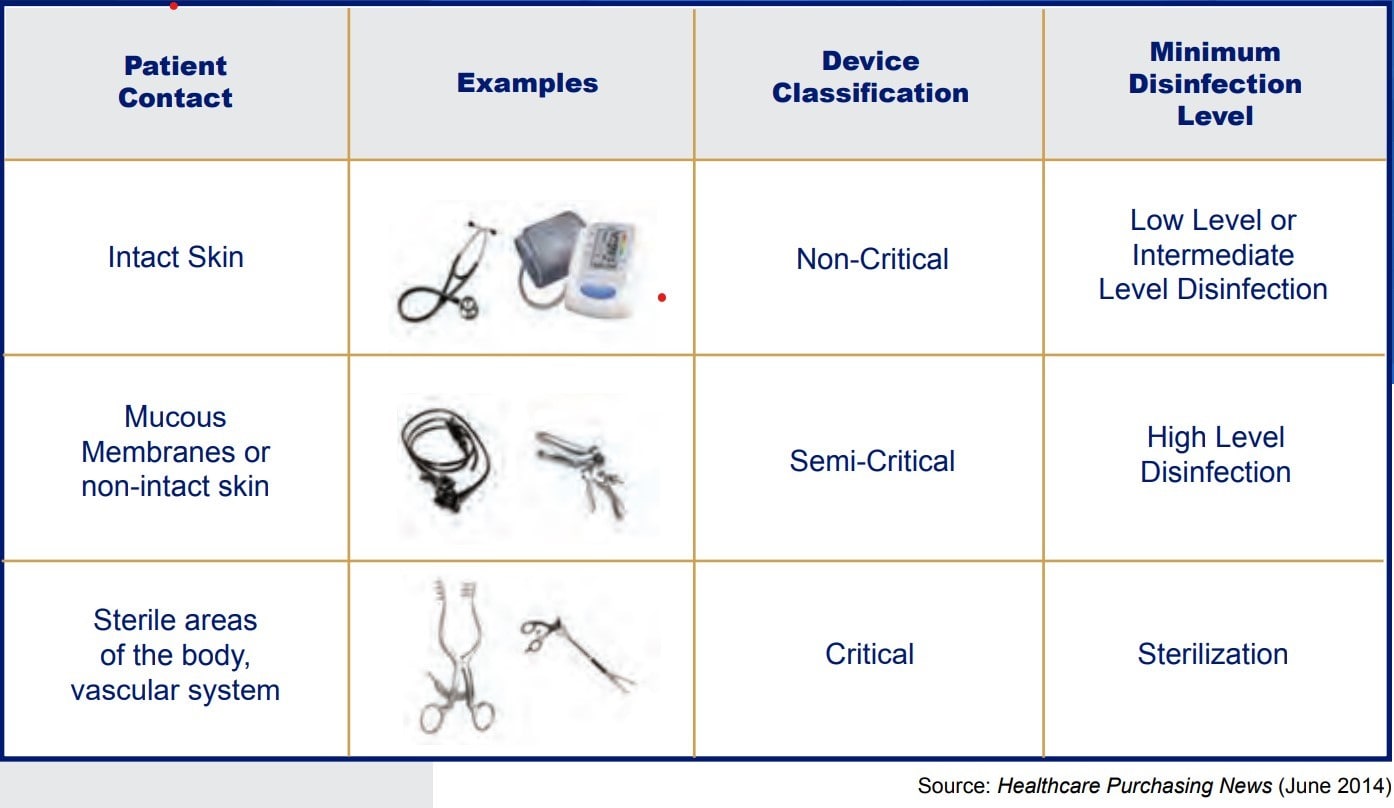
 More than 45 years ago, Earle H. Spaulding developed an approach to disinfect and sterilize patient-care items and equipment. His approach has been successfully used by infection control professionals and others when planning methods for disinfection or sterilization. Spaulding believed that if instruments and devices for patient care were placed into different classifications according to the degree of risk for infection involved in use of the items, this would give guidance as to how to disinfect and sterilize them. Below is the classification system he devised, which has come to be known as the “Spaulding Classification”.
More than 45 years ago, Earle H. Spaulding developed an approach to disinfect and sterilize patient-care items and equipment. His approach has been successfully used by infection control professionals and others when planning methods for disinfection or sterilization. Spaulding believed that if instruments and devices for patient care were placed into different classifications according to the degree of risk for infection involved in use of the items, this would give guidance as to how to disinfect and sterilize them. Below is the classification system he devised, which has come to be known as the “Spaulding Classification”.
Spaulding Classification
When talking about high level disinfection we are looking at those items in the semi-critical section of the Spaulding Classification grid. Semi-critical items are those that come in contact with mucous membranes or nonintact skin. Some examples of these include, but are not limited to, respiratory therapy and anesthesia equipment, some endoscopes, vaginal probes, and laryngoscope blades. These medical devices should be free from all microorganisms; however, small numbers of bacterial spores are permissible since the lungs and gastrointestinal tract, generally are resistant to infection by common bacterial spores but susceptible to other organisms, such as bacteria, mycobacteria, and viruses.
Semi-critical items minimally require high level disinfection using chemical disinfectants. Glutaraldehyde, hydrogen peroxide, and ortho-phthalaldehyde (OPA), are cleared by the Food and Drug Administration (FDA) and are dependable high-level disinfectants. When a disinfectant is selected for use with certain patient-care items, the chemical compatibility after extended use with the items to be disinfected also must be considered. Before utilizing any high-level disinfectant always reference the manufacturer’s information for use (IFU) for the disinfectant as well as the device.
Steps to clean semi-critical classified items
Regardless of the solution you use, always remember these key steps:
• Follow manufacturer instructions for use (IFUs).
• Monitor the HLD, documenting and dating the solution per the IFU.
• Test the solution for minimum effective concentration
• Discard the solution when it is no longer effective.
• Follow labeling directions for contact time, temperature and soak time
• Document your quality control testing.
When using a high-level disinfectant, it is important to remember to follow the recommended process in the IFU and use the personal protective equipment required. Before the scope or device is received in the reprocessing areas it should be pre-cleaned by wiping scope or device down with an enzymatic solution at the point of use. This removes any blood, bioburden and/or body fluids that may adhere to the scope or device making it challenging to remove. Once pre-cleaned the scope or device is sprayed with a recommended enzymatic solution or covered with a moist towel and transported in OSHA approved container that is leak-proof and puncture-proof that contains a biohazard label.
The reprocessing area is where all equipment for cleaning devices is housed. This area contains sinks for cleaning, soaking and rinsing your scopes or devices as well as ultrasonic equipment and washers. Here you should don your personal protective equipment (hair cover or cap, face mask with eye shield, fluid resistant gown and durable gloves) prior to receiving the cart or container of contaminated devices from the Procedure or Operating Room.
Once received in the reprocessing area it should be leak tested, if applicable, and manual cleaning performed. The scope or device should then be rinsed and disinfected either manually or in an automated washer per the IFU. Finally, it should be rinsed, dried and stored per the IFU.
In summary, high level disinfection ensures the safe use of devices that are classified as semi-critical according to the Spaulding Classification grid. However, it is important to remember that before cleaning and/or disinfecting any device, refer to the manufacturer’s information for use to ensure effective disinfection and avoid damage to those devices.
REFERENCES
William A. Rutala, M. D. (2013). Disinfection and sterilization: An Overview. American Journal of Infection Control, 52-55.
High Level Disinfection (HDL) and Sterilization BoosterPak, The Joint Commission (2015)
HICPAC: Guideline for Disinfection and Sterilization in Healthcare Facilities (2008)
Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the Healthcare Infection Control Practices Advisory Committee (2017)
Infection Control Today, High-Level Disinfection: AORN Issues Updated Guidance (April 19, 2018)

