There are many aspects to an accreditation survey, therefore an organization must perform assessments in all areas to prepare for continuous survey readiness. One important subset in the perioperative area is anesthesia services. Anesthesia can be engaged across several standards including environment of care, infection control, leadership, medical staff, medication management, process improvement, provision of care and record of care. Of these, medication management, environment of care, infection control, and provision of care are four areas that are frequently cited for anesthesia.
Medication Management
- Finding: Based upon review of the anesthesia cart contents, there were pre-filled, unlabeled syringes of medications in 2 of the 4 carts reviewed
Per National Patient Safety Goal (NPSG) 3 (Improving the safety of using medications), medications on and off the perioperative field that are not being immediately administered must be labeled with:
a) Medication or solution name
b) Strength
c) Amount of medication or solution containing medication (if not apparent from the container)
d) Diluent name and volume (if not apparent from the container)
The definition of immediate administration is a medication that an authorized staff member prepares or obtains, takes directly to a patient, and administers to that patient without any break in the process. Therefore, if it is held for later use, it must be labeled! The date and time are not required if held less than 24 hours or as defined by the hospital policy.
The American Society of Health-System Pharmacists (ASHP) updated the Guidelines on Perioperative Pharmacy Services in 2019 to reflect common medication safety practices (https://library.smh.com/sites/default/files/ASHP%20guidelines%20on%20perioperative%20pharmacy%20services.pdf). If your facility does not have a dedicated perioperative pharmacist, there are things your organization can do to encourage safe medication practices by your anesthesia team.
- Automated dispensing systems to help with accountability, storage, accuracy, and timeliness
- Limiting access and making sure carts can be secured especially after hours (see Medication Security-Anesthesia Cart Joint Commission FAQ https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medication-management-mm/000001526/)
- Use primarily single-dose vials; discard multi-dose vials at end of every case; use only preservative-free local anesthetic products
- Stock only 1 drug concentration on cart; include an alert label on concentrated or high-alert drugs
- Standardize medication trays, clearly label sections, and place drugs to minimize confusion and hidden labels
- Store regional anesthesia drugs in a separate regional cart
- Label all medications using standardized preprinted labels or labels generated by barcode scan
- Minimize provider-prepared syringes when possible; use prefilled syringes and premixed intravenous (i.v.) solutions when possible
- Encourage anesthesiology department representation on the medication safety committee(s), keeping in mind that crucial differences between anesthesia medications and those used elsewhere may warrant a separate anesthesia medication safety committee.
Environment of Care/Infection Control
- Based upon review of 4 of 4 anesthesia machines, there was no evidence present to substantiate that the daily machine checks were being completed.
- Based upon review of 1 of 5 anesthesia machines, the carbon dioxide absorbent was dirty and crusted.
Environment of Care and Infection control often go hand in hand during a survey. The perioperative area is considered a critical area; therefore, it is closely scrutinized for cleaning processes and preventive maintenance. It is imperative to review instructions for use (IFUs) and equipment manuals for maintenance schedules. Often, newer anesthesia machines have daily checklists that can be set to run at the beginning of the day. Your anesthesia team members should be able to speak to when the tests are completed and how (automated versus manual). Your policies should align with the abilities of the equipment and may need to reflect both a manual check process and automated check process if you have a variety of anesthesia machines.
Manuals will also contain information on how to properly clean and disinfect particular pieces of the anesthesia machine. Organizations have begun to master how to disinfect and store laryngoscope blades and handles but have not considered the anesthesia machine itself. There are preventive maintenance items that should be completed by the anesthesia team. The team should be aware if there are requirements such as “change CO2 absorbers every eight hours of use”. Here is an example schedule of cleaning and maintenance for a Drager anesthesia machine:
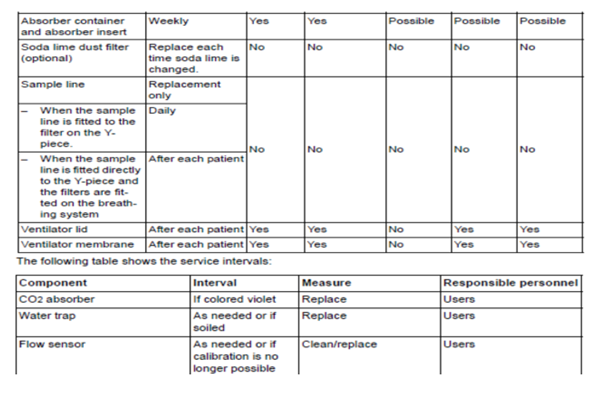 https://www.draeger.com/Library/Content/IfU_Fabius_GS_premium_SW_3n_9054626.pdf
https://www.draeger.com/Library/Content/IfU_Fabius_GS_premium_SW_3n_9054626.pdf
Provision of Care
The Centers for Medicare and Medicaid State Operations Manual (CMS SOM_A) and Leadership standard 04.01.05 EP 9 states anesthesia is responsible for all anesthesia that is completed within the hospital. The anesthesia department must be led by a qualified doctor of medicine or osteopathy unless exempted by the state (see CMS SOM_A §482.52(c)). Medical staff and the governing body should be reviewing these qualifications. If the department is contracted, there should be a clear delineation of the requirements of the department. The organization’s performance work statements or key performance indicators may clarify quality points that are to be reviewed and the frequency of review.
Some quality measures that can be self-assessed are pre and post-anesthesia evaluation requirements. Please note that a pre-sedation assessment (for moderate sedation) is different from a pre-anesthesia requirement. From CMS SOM_A 2/20 “ a pre-anesthesia evaluation performed by someone qualified to administer anesthesia as specified in §482.52(a) is not required because moderate sedation is not considered to be “anesthesia”, and thus is not subject to that requirement under this regulation.” This is because moderate sedation is not considered anesthesia, but analgesia. Deep sedation is considered a part of anesthesia.
Please also review CMS SOM_A for requirements to assess items such as your post-anesthesia assessments. Note: If a patient is unable to participate in a post-anesthesia assessment, this should be noted along with these required elements:
- Cardiovascular function, including pulse rate and blood pressure
- Respiratory function, including respiratory rate, airway patency, and oxygen saturation;
- Mental status;
- Temperature;
- Pain;
- Nausea and vomiting; and
- Postoperative hydration.
This assessment as well as those noted below are typical medical record reviews during a survey.
| Medical Record Review Components | TJC Standard | A Tag/CoP |
| Complications HAIs and unfavorable reactions to drugs and anesthesia | RC 02.01.01 EP 2 RC 02.01.03 EP 8 | A0465 – 482.24(c)(4)(iv) |
| Pre-anesthesia eval within 48 hours prior to surgery or anesthesia | PC 03.01.03 EP 18 | A1003 – 482.52(b)(1) |
| Intraoperative anesthesia record or report | PC 03.01.05 EP1 RC 02.01.03 EP 1 | A1004 – 482.52(b)(2) |
| Post anesthesia eval no later than 48 hours after surgery or anesthesia | PC 03.01.07 EP 7 8 | A1005 – 482.52(b)(3) |
(TJC Survey Activity Guide, 2022)
Lastly,be certain to observe your Universal Protocol timeouts to make sure that everyone, inclusive of anesthesia, are providing their undivided attention and actively participating in the time-out.
Anesthesia services is an integral part of your perioperative team. Engaging them in your assessments and actual surveys is essential for successful surveys, but most importantly for patient safety.
To learn more about Anesthesia Services survey readiness contact the C&A team at 704-573-4535 or email us at info@courtemanche-assocs.com.


